Ethyleneoxide, propyleneoxide and epoxybutene can be produced in a cooled tubular reactor. This requires partial oxidation of ethylene, propylene and butadiene respectively. The complete oxidation of the reactants to CO2 and H2O is an undesired side reaction.
The kinetics can be simplified to:
desired) a A + b1 B --> p P + p dHP (R1 = A01 exp(-Ea1/RT) cAa1 cBb1)
undesired) a A + b2 B --> x X + x dHX (R2 = A02 exp(-Ea2/RT) cAa2 cBb2)
Both gasphase reactions are (strongly) exothermic. For the design of an optimal and safe reactor (no runaway) it's necessary to be able to simulate both concentration and temperature profiles. Therefore a Pascal program was written to solve four mass balances (plug flow with axial dispersion) and an integrated heat balance simultaneously. The program can calculate stationary as well as instationary profiles and it can also be used to perform runaway analysis.
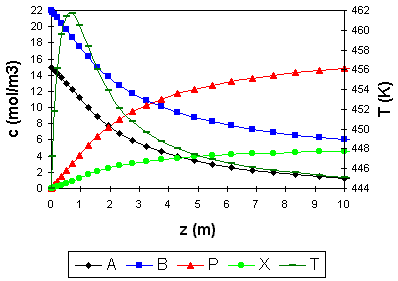
An example of a stationary profile is given in the figure.
