![]()
One of the products of a hydrocracker is 1,3-butadiene, which can be used for the production of 1,4-butanediol, a chemical with good market expectations. The conversion of 1,3-butadiene to 1,4-butanediol requires several steps. The first step is the epoxidation of 1,3-butadiene to 3,4 epoxybutene (EpB). Two production methods of EpB have recently been patented: The Eastman process using oxygen and the Enichem process using hydrogenperoxide.
From a technical point of view the Enichem process is an interesting process. Itís a liquid phase process instead of a gas phase process and therefore requires smaller equipment. The reaction will probably have a higher yield and the process is safer. From a black box comparison of the two mentioned processes (50,000 tons EPb/year) it can however be concluded that the Eastman route (oxygen based) is much more profitable. This is mainly caused by the fact that oxygen is much cheaper then hydrogenperoxide. The price of hydrogenperoxide will at least have to sink to 0.62 $/kg (current price is about 1.0 $/kg) to make the Enichem route remunerative.
After the blackbox evaluation the design of the Eastman process has been continued with a conceptual design. At this design level the use of air and oxygen is compared. It is shown that the use of air is not a good choice. Itís also shown that a diluent is required to remove the heat of reaction in the cooled tubular reactor. A comparison between nine diluents shows that n-butane is the best diluent, because of its high molar heat capacity.
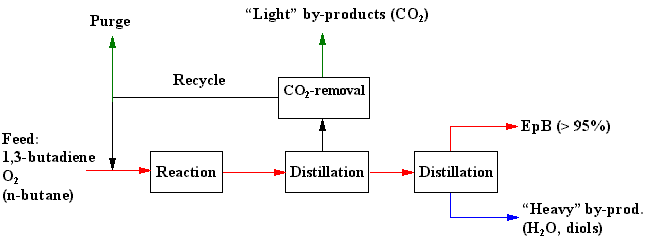
Based on the process and equipment design the following optimal design and operation specifications are obtained: